The Vibamat
A revolution
in medical technology

- Anonymous or personalised tests possible
- Test result within 20 seconds
- No waste products
- Listed as a medical device by the Federal Institute for Drugs and Medical Devices (BfArM)
- 100% developed and produced in Germany

The Vibamat
Optical measurement of viruses and
Bacteria in the air we breathe

A NEW ERA
The Vibamat detects the viral or bacterial load in the breath in the sub-nanometre range with the help of an antibody-coated semiconductor chip and a highly sensitive laser.
Result in 20 seconds
Detection of viruses or bacteria - in just 20 seconds. The Vibamat also detects the pathogen load of the lower respiratory tract, i.e. the lungs.


625 BREATH TESTS PER CHIP CARD
A single antibody smart card can be reused up to 625 times. This avoids plastic waste and protects the environment. Testing with the Vibamat is also cheaper than other methods.
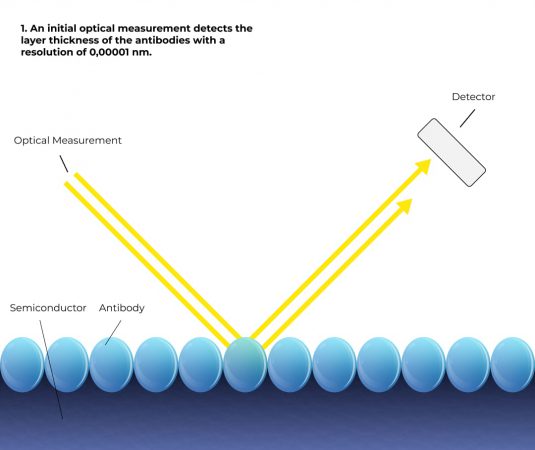
How it works
Simplified representation
Based on 6 steps
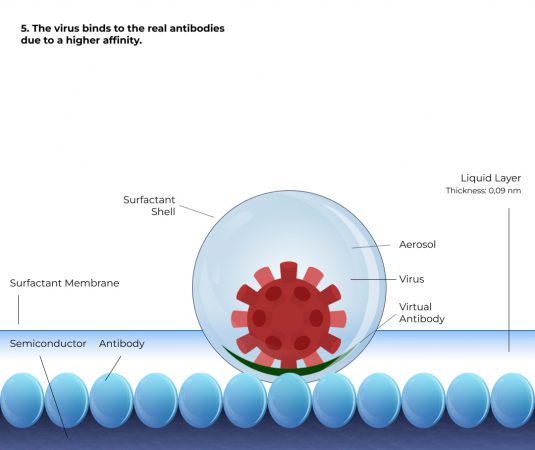
Comparison
of test methods
EARLY DETECTION EVEN
BEFORE SYMPTOMS
The results of conventional tests often vary because the pathogen load is determined directly on the mucous membrane in rapid tests and PCR tests. The Vibamat, on the other hand, achieves unambiguous results: by measuring the viral or bacterial load in the breath, an infection can be detected even before symptoms appear. A meaningful result is then available within 20 seconds.
The technology
Worldwide patented
Technology
According to the invention, there are two different methods to generate significant burst kinetics, a phase coupling of antigens via their charges and a mechanical phase coupling. The Vibamat relies on a new type of substrate materials for phase coupling via charges. These are materials that like to oxidise and are also very flat. These can be, for example, smooth silicon or aluminium substrates. With both materials, an oxide layer forms quickly. With silicon, the Si02 oxide layer grows by approx. 0.7 nm within the first hour. With aluminium, automatic oxidation also takes place. In electrical phase coupling, a substrate is first made oxide-free or the oxide layer is significantly reduced in order to couple antigens. Oxide-free or oxide-reduced silicon can be obtained, for example, by removing the oxide layer from a silicon wafer with an SiO2 layer using hydrofluoric acid. The antigens are coupled to the oxide-free or oxide-reduced silicon wafer by applying antigens in aqueous solution. In the case of aluminium, aluminium foils can be used as a substrate. During the manufacturing process, the foils are rolled in a folded state, whereby the inner sides of the folded foils are largely oxide-free. Only when the foils are pulled apart does oxidation begin. If antigens are placed on the foils at this point, they adhere instantly. In order to obtain phase coupling via charges, the distance between the antigens and each other must be so small that the charges of the antigens can interact with each other.
Antigens have positive charges in the form of NH3+ ions and negative charges in the form of COO- ions, the isoelectric point being between the positive and negative charges. In addition, antigens have at least one beta-sheet or alpha-helix. It is well known that both beta - leaflets and alpha - helices behave like linear or non-linear springs. Furthermore, it is known that the structures oscillate in the terahertz range. By applying the mathematical methods of engineering mechanics and using Maxwell's equations, it can be proven that phase coupling of the antigen oscillations occurs automatically if the antigens are arranged close enough to each other. The phase coupling is particularly strong if a plane can be placed through all isoelectric points of the antigens. Therefore, the substrate should be very flat. The coupled oscillating antigens generate a resulting charge shift field by superposition of the individual fields, which is larger than the respective charge shift field of an individual antigen. The range of the charge-shift waves increases with the square root of the number of coupled antigens. It is therefore necessary for a rapid test to have a sufficient number of antigens coupled in the phase.
An elastic membrane with antigens is placed on a smooth silicon substrate with or without an oxide layer. The membrane can be cell or virus membranes with epitopes. A water monolayer is automatically formed between the lipid membrane and the substrate surface, which allows the membrane to oscillate, thus enabling phase coupling of the antigens. After the antigen layer is formed, blocking by molecules or by the natural oxidation process occurs. Natural blocking by oxidation can occur via a natural or artificial oxidation process. In the natural oxidation process, the natural oxygen in the air is used; in the artificial oxidation process, additional oxygen is added. Unfortunately, SiO2 is hydrophilic, but it can be made hydrophobic. To do this, the SiO2 layer can simply be exposed to normal air. After a certain time, a hydrophobic layer (4) forms on the SiO2. This process can be shortened by evaporating oil or grease in the vicinity. A hydrophobic thin film then forms on the SiO2. This hydrophobic thin film improves blocking.
A liquid layer containing the biomolecules to be analysed must be present above the antigen substrate. However, the liquid layer must only be thick enough for the charge displacement waves to penetrate the layer with sufficient strength. The field strength must be so great that the biomolecules, e.g. enzymes, antibodies, viruses, bacteria or cells, can detect where the antigens are located at any point in the liquid layer. They will then automatically move towards the antigens to dock with them. The thinner the layer, the faster the coupling process takes place. If the above framework conditions are fulfilled, then all biomolecules couple, i.e. there is a clear deviation from the law of mass action. The non-linear kinetics of the mass action law is replaced by a controlled process with linear kinetics. This controlled process is significantly faster compared to the mass action law and enables the development of rapid tests. Experimentally, a range of about 2 mm could be identified in which antibodies and viruses recognise and swim towards their antigens, and similar can be expected for bacteria and cells. It was also experimentally proven that in this range, both antibodies and viruses swim towards the substrate at a constant speed. Experimentally, a speed of about 2 mm/s could be determined for IgE antibodies; for viruses, the speed is 0.1 - 0.2 mm/s.
With a liquid column of 1 mm above the antigen-coated substrate, all antibodies from the liquid column have docked within 0.5 seconds. For viruses, the time is about 5 to 10 seconds. Instead of a continuous liquid column, aerosols containing biomolecules can also be measured. With an aerosol diameter of 1 micrometre, viruses require a period of 5 to 10 milliseconds until they have docked.
In order to be able to measure aerosols, the substrate must be colder than the aerosol. This causes the aerosols to condense on the antigen layer and leads to the coupling process. The metrological evaluation can be carried out according to US6168921. Using this technology, a layer thickness change of 20 femtometres can be detected. Although the individual biomolecules are larger, the optical diffraction limitation means that average layer thicknesses of less than one atomic diameter can be measured. If a 160 nm virus with a volume of 2.1 * 10-3 µm3 is coupled to a measurement area of 3 mm2 , this corresponds to an average layer thickness increase of 0.71 femtometres on the 3 mm2 substrate area. With an instrumental resolution of 20 femtometres, the method then has a resolution of 28 viruses, for example. The resolution can be further increased by using a camera as a detector. Even if the signal noise of the camera is higher by a factor of 10,000 than with the method without a camera and the layer thickness resolution of each pixel is thus only 0.2 nm, a single virus can be identified very well with a diffraction limit (lateral resolution) of 1 µm. The resolution limit of the method is then about 1/10 virus.
If the substrate is very smooth, then the antigen layer on the substrate does not produce significant scattered light. If larger molecules such as viruses, bacteria or cells couple to the antigens, then this will generate scattered light. Even molecules smaller than the diffraction limit produce scattered light that can be seen with the naked eye or optical aids (e.g. imaging optics with a camera). Experiments have shown that as small as 40 nm bioparticles can be seen with the naked eye. This is possible because the scattered light has a divergent beam path.
Our
History
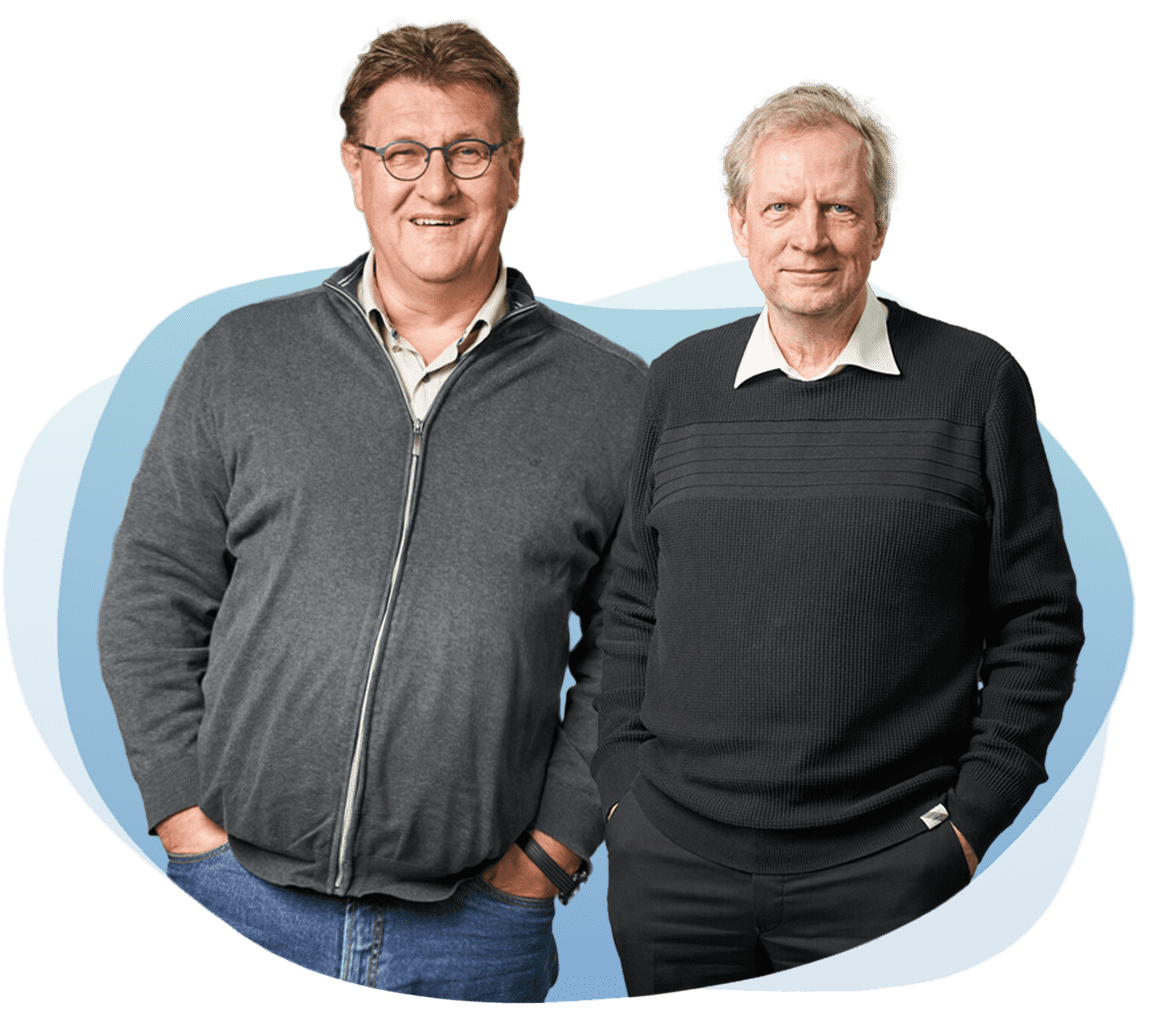
Dr.-Ing. Udo Riss
and Michael Bubolz
Together for sustainable and efficient corona testing - that is our mission. With innovative technology Made in Germany and bundled expertise from science and technology, we achieved a breakthrough in the field of SARS-CoV-2 diagnostics about a year ago.
Contact us
Just send us
your request
If you are interested, please call us or use our form. We are currently receiving a large number of enquiries, which may result in longer waiting times. We ask for your understanding.

By submitting the form, you agree to your data being processed for the purpose of responding. You can find the data protection regulations here.
We are available for you by telephone from Monday to Friday from 8am to 6pm!
Use our e-mail service for your enquiry!
Please use the contact form for all press enquiries, interviews and other matters:
